
Full text loading...
Tight junctions, paracellular permeability barriers that define epithelial cell polarity, play an essential role in transepithelial transport, cell–cell adhesion and lymphocyte transmigration. They are also important for the maintenance of innate immune defence and intestinal antigen uptake. Ammonium ( ) is elevated in the gastric aspirates of Helicobacter pylori-infected patients and has been implicated in the disruption of tight-junction functional integrity and the induction of gastric mucosal damage during H. pylori infection. The precise mechanism of the effect of ammonium and the molecular targets of ammonium in host tissue are not yet identified. To study the effects of ammonium on epithelial tight junctions, the human colon carcinoma cell line Caco-2 was cultured on permeable supports and the transepithelial resistance (TER) was measured at different time intervals following exposure to ammonium salts or H. pylori-derived ammonium. A biphasic response to treatment with ammonium was found. Acute exposure to ammonium salts or NH3/
) is elevated in the gastric aspirates of Helicobacter pylori-infected patients and has been implicated in the disruption of tight-junction functional integrity and the induction of gastric mucosal damage during H. pylori infection. The precise mechanism of the effect of ammonium and the molecular targets of ammonium in host tissue are not yet identified. To study the effects of ammonium on epithelial tight junctions, the human colon carcinoma cell line Caco-2 was cultured on permeable supports and the transepithelial resistance (TER) was measured at different time intervals following exposure to ammonium salts or H. pylori-derived ammonium. A biphasic response to treatment with ammonium was found. Acute exposure to ammonium salts or NH3/ derived from urea metabolism by wild-type H. pylori resulted in a 20–30 % decrease in TER. After 24 h, the NH4Cl-treated cells showed a partial recovery of TER. In contrast, the control culture, or cultures that were exposed to supernatants derived from urease-deficient H. pylori, showed no significant decrease in TER. Occludin-specific immunoblots revealed the expression of a low-molecular-weight form of occludin of 42 kDa upon NH3/
derived from urea metabolism by wild-type H. pylori resulted in a 20–30 % decrease in TER. After 24 h, the NH4Cl-treated cells showed a partial recovery of TER. In contrast, the control culture, or cultures that were exposed to supernatants derived from urease-deficient H. pylori, showed no significant decrease in TER. Occludin-specific immunoblots revealed the expression of a low-molecular-weight form of occludin of 42 kDa upon NH3/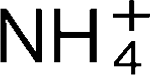 exposure. The results indicate that modulation of tight-junction function by H. pylori is ammonium-dependent and linked to the accumulation of a low-molecular-weight and detergent-soluble form of occludin.
exposure. The results indicate that modulation of tight-junction function by H. pylori is ammonium-dependent and linked to the accumulation of a low-molecular-weight and detergent-soluble form of occludin.